Regulatory Services
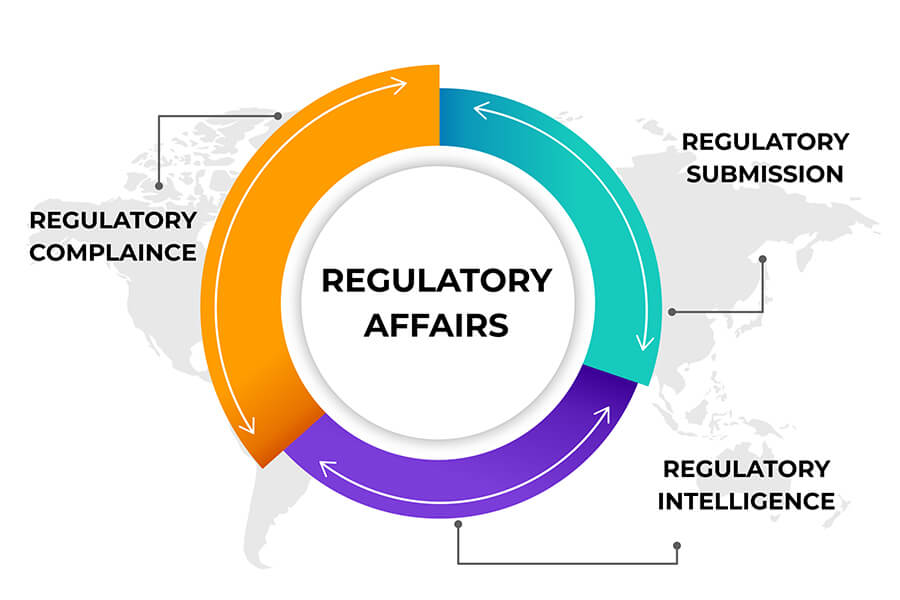
In today’s competitive environment, the reduction of the time taken to reach the market is critical to a product and hence the company’s success. The proper conduct of its Regulatory Affairs activities is therefore of considerable economic importance for the company.
Regulatory Affairs have been developed from the desire of Government to protect public health by controlling the safety and efficacy of products in areas including pharmaceuticals, nutraceuticals etc.. DPS Consultants consistently produces high-quality documents that move pharmaceutical companies and other client types to the desired outcome more quickly and efficiently.
SUPPORTIVE SERVICES
- Handling all MoH Queries (till product registration)
- Review Dossier as per Updated ASEAN guideline
- Agreements (CDA/NDA & Supply) Writing & Review
- Separate DMF (in CTD / ACTD format)
- More than 500 ACTD Dossier templates (Ready-to-write: to save time)
- Reference ACTD Dossiers / DMFs / BE Database